Regulating The Bacterial Photosystem In Response To Changes In Oxygen Tension
The RegA-RegB global regulatory cascade
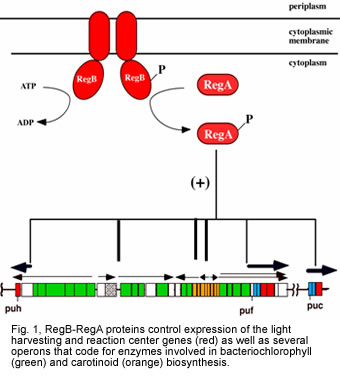 Much of our efforts on the regulation of the bacterial photosystem have been centered on the two component regulators RegB-RegA from Rhodobacter capsulatus. RegB is a membrane-bound redox responding spanning sensor kinase and RegA is a DNA binding response regulator.
Much of our efforts on the regulation of the bacterial photosystem have been centered on the two component regulators RegB-RegA from Rhodobacter capsulatus. RegB is a membrane-bound redox responding spanning sensor kinase and RegA is a DNA binding response regulator.
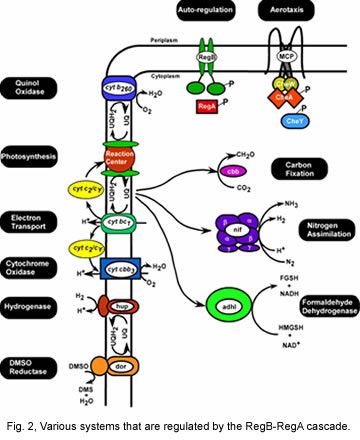
After identifying these components as major regulators of the photosystem, we subsequently established that the RegB-RegA cascade functions as global regulators of numerous physiological processes in R. capsulatus. For example, we have demonstrated that RegB-RegA controls synthesis of the photosystem, components of the respiratory chain including various cytochromes, ubiquinone etc, the hydrogenase complex, components of the carbon and nitrogen fixation pathways and various dehydrogenases. These studies involved both gene expression analyses in mutant cell lines as well as DNA binding studies on various promoters with isolated RegA (reviewed in Elsen et al., 2004; Wu and Bauer 2008; Wu >et al>., 2012).
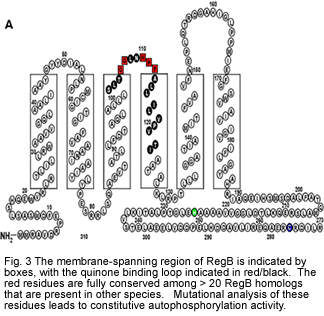
Our recent efforts on the RegB-RegA system have been centered on biochemical analysis of redox sensing by RegB. Recent studies have demonstrated that RegB binds both oxidized and reduced ubiquinone with oxidized ubiquinone inhibiting kinase activity. Equilibration of RegB with the ubiquinone pool allows RegB to monitor the redox state of the ubiquinone pool and to regulate kinase activity accordingly (Swem et al., 2006; Wu and Bauer, 2010). We have also determined that part of the regulation of RegB kinase activity is controlled by formation of a cysteine sulfenic acid (Swem et al., 2003; Wu et al., 2013). Currently, we are utilizing a variety of structural methodologies to define how binding of oxidized quinones inhibits autophosphorylation activity of RegB.
The CrtJ response
In addition to RegB-RegA, R. capsulatus also contains a second redox responsive regulatory system known as CrtJ (also called PpsR in some species). CrtJ/PpsR functions as a repressor of heme, bacteriochlorophyll, carotenoid, light harvesting and cytochrome gene expression (Fig. 4).
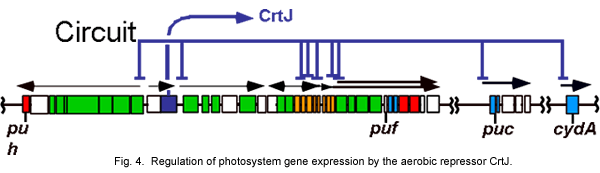
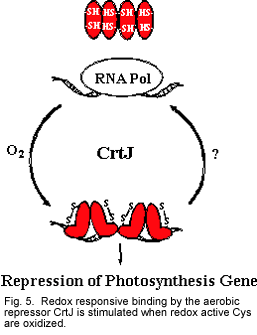
Mutational and biochemical studies with isolated CrtJ/PpsR have demonstrated that this protein contains a redox active cysteine (Cys) that affects its DNA binding activity (Masuda et al.,2002; Masuda and Bauer 2005; Cheng et al., 2012). When the redox active Cys is oxidized, it stimulates binding of CrtJ to target promoters and when it is reduced it inhibits binding. Our recent studies have demonstrated that when exposed to oxygen CrtJ forms a cysteine sulfenic acid that stimulates its DNA binding activity.
We also undertake comparative studies of a CrtJ homolog in R. sphaeroides called PpsR (Masuda et al., 2002, Yin et al., 2011). Ppsr also contains a redox active Cys, and in addition, binds heme (Yin et al., 2011). Interestingly, one of the ligands for binding heme is the same redox active Cys as described for CrtJ. The binding of heme by PpsR affects its DNA binding activity and its ability to regulate heme versus bacteriochlorophyll gene expression (Yin et al., 2012).
Relevant References:
Recent reviews
Wu, J., V. Dragnea & C.E. Bauer. 2012. Redox Responding Sensor Kinases. In Two Component Systems in Bacteria. Ed. R Gross and D Beier. pp 41-56. Horizon Scientific Press.
Wu, J., & C. E. Bauer. 2008. RegB/RegA, A Global Redox-Responding Two-Component Regulatory System. In, Bacterial Signal Transduction: network and drug targets. Ryutaro Utsumi edt. Advances in Experimental Medicine and Biology vol 631 pp 131-148, Landes Bioscience Eurekah, Georgetown, TX
Elsen, S., L. Swem, D. Swem, & C. E. Bauer. 2004. RegB/RegA, a highly conserved redox-responding global regulatory system. Microbio. and Mol. Biol. Rev. 68, 263-279
Redox papers
Yin, L., V. Dragnea, G. Fieldman, L. A. Hammand, J. A. Karty, C. Dann, & C. E. Bauer. 2013. Redox and light control of the heme sensing activity of AppA. mBio 4: e00563-13
Wu J., Cheng, Z., Reddie, K., Carroll, K., Hammad, L.A., Karty, J.A., & C. E. Bauer. 2013. RegB kinase activity is repressed by oxidative formation of cysteine sulfenic acid. J. Biol. Chem. 288:4755-4762. PMID: 23306201
Yin, L., V. Dragnea, & C. E. Bauer. 2012. PpsR, a regulator of heme and bacteriochlorophyll biosynthesis, is a heme-sensing protein. J. Biol Chem. 287:13850-13858. PMID: 22378778
Cheng, Z., J. Wu, A. Setterdahl, K. Reddie, K. Carroll, L. A. Hammad, J. A. Karty & C.E. Bauer. 2012. Activity of the tetrapyrrole regulator CrtJ is controlled by oxidation of a redox active cysteine located in the DNA binding domain. Mol. Micro. 85:734-746 PMID: PMC3418406
Yin, L., V. Dragnea, & C. E. Bauer. 2012. PpsR, a regulator of heme and bacteriochlorophyll biosynthesis, is a heme-sensing protein. J. Biol. Chem. 287:13850-13858. PMID: 22378778
Wu J, & C.E. Bauer. 2010. RegB kinase activity is controlled in part by monitoring the ratio of oxidized to reduced ubiquinones in the ubiquinone pool. MBio. 1. pii: e00272-10.
Swem, L. R., X. Gong, C.-A. Yu & C. E Bauer. 2005. RegB controls a diverse array of metabolic processes by monitoring the redox state of the ubiquinone pool. J. Biol. Chem. 281, 6768 - 6775.
Smart, J. L., Willet, J., & C. E. Bauer. 2004. Identification of multiple regulators controlling heme biosynthesis in Rhodobacter capsulatus J. Mol. Biol . 342, 1171-1186
Swem, L., B. J. Kraft, D. L. Swem, A. T. Setterdahl, S. J. Masuda, D. B. Knaff , J. M. Zaleski & C, E, Bauer. 2003. Signal Transduction By The Global Regulator RegB Is Mediated By A Redox Active Cysteine. EMBO J. 22, 4699-4780
Masuda, S., Dong, C., D. Swem, A. T. Setterdahl, D. B. Knaff , & C. E. Bauer. 2002. Aerobic repression of photosystem synthesis through formation of an intramolecular disulfide bond in CrtJ. Proc. Natl. Acad. Sci. USA 99, 7078-7083
Masuda, S. & C. E. Bauer. 2002. AppA is a blue-light photoreceptor that anti-represses photosynthesis gene expression in Rhodobacter sphaeroides Cell 110, 613-623. (Featured on cover)
